Looking for an ACP? Have No Importer in Japan? We are Here to Help You!
Electric Appliances
How to Import PSE-Regulated Electric Appliances
Electrical Appliances and Material Safety Act (PSE Act)
Many electric appliances are regulated by Product Safety of Electrical Appliances and Materials Act (PSE Act). Japanese importer must notify to the Ministry of Economy, Trade and Industry(METI) in order to sell PSE-regulated appliances. Importers serve as manufacturers in terms of the product liability, and have to conduct quality control and tracking of each unit sold. Inspection prior to sales is not sufficient, and the importers have to ensure that each lot is safe and compliant, and be ready for any dispute or legal actions in case of any product deficiency.
PSE Application can Only be Submitted by Japanese Companies.
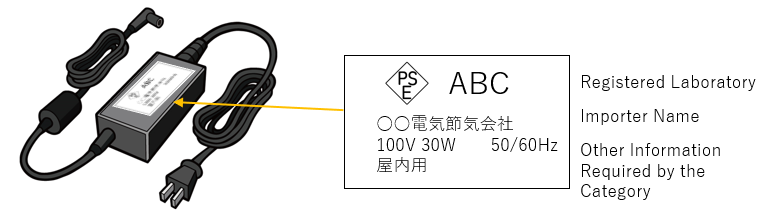
PSE marks are proof of safety confirmation and assurance by the notifier, not a certification. These marks are only allowed to be displayed by Japanese entities, and for imported products generally come with the mark with the importer’s information. If a non-resident importers want to import PSE-subject items, the PSE procedures can be handled by a Japanese company that such non-resident entity have appointed. Therefore, it is essential to either find a partner that provides such services or appoint an ACP (Accredited Customs Broker) capable of handling this."
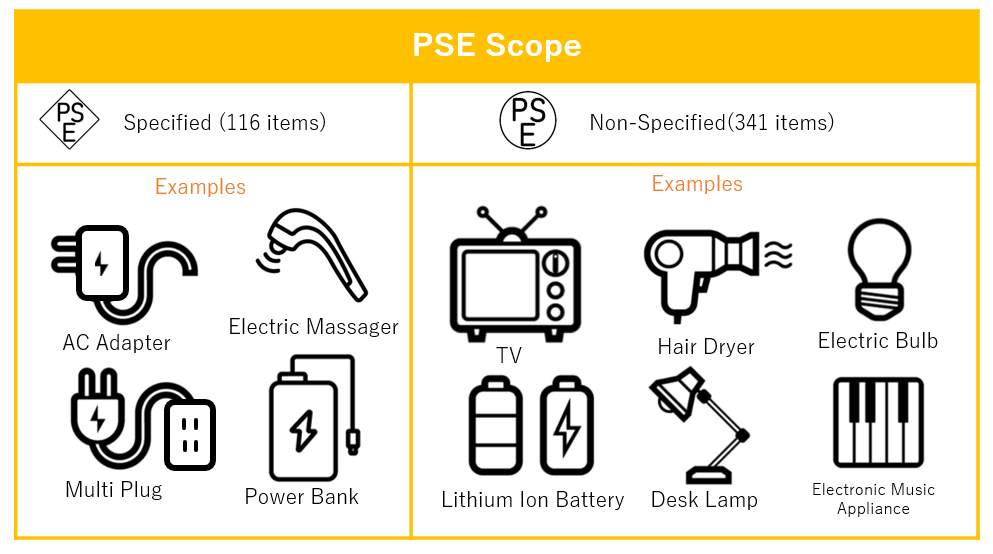
PSE Scope
For quick reference, here are the basic items regulated by PSE Act.
- Electric appliance with AC adapters
- Mobile battery containing cell of which energy density is not less than 400Wh/L
- Lithium ion battery which energy density is not less than 400Wh/L
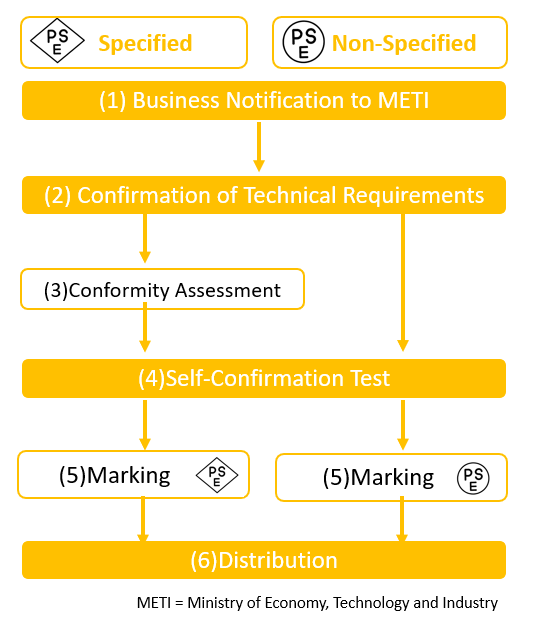
Import Process
User Manual and Marketing


Manufacturers and importers are either obliged or advised to state the following information on electric appliance labels, subject to Household Quality Labeling Act and/or Fair Competition Agreement.
- Company name and address
- Product name and description
- Specification
- The period for which the repair parts remains available
- The conditions that may influence consumers' purchase decision, i.e., the place where the appliance can be installed, the cost of installment, the condition for use, warranties, etc..
Subject to Act for Promotion of Use of Recycled Resources, the packages also must come with recycling marks.
Expected Costs and Timeline

Notification and quality control : 1,000,000 JPY~/Product
Required Time : 6 to 12 months or more
<PSE Act> Other Possible Concerning Laws
Juicers, coffee makers and other electric appliances with food contact are regulated by Food Sanitation Act. Check here to learn more about Food Sanitation Act.
<PMD Act> Other Possible Concerning Laws
PMD Act governs distribution of drugs, quasi-drugs, cosmetics, medical devices, etc.. Some electric massagers and beauty device may be regulated by this law, subject to the purpose and function of the device as well as the description on the marketing materials.
Importing and selling medical devices requires pharmaceutical license, which is only granted to qualified companies located in Japan.
Visit this page to learn more about PMD Act.
<IP Laws> Other Possible Concerning Laws

It is prohibited to import items that infringe other people's patent, utility model rights, trademarks, or copyrights. Make sure that your merchandise is authentic, legitimate and authorized to sell in Japan.
<Radio Act> Other Possible Concerning Laws
Telephones, modems, Bluetooth devices and other appliances that emit electric waves are regulated by Radio Act in Japan. They must get tested for technical quality conformity by registered certification bodies, and come with what's called Giteki mark and conformity numbers on the labels.
This law was originally intended to control radio stations (commercial, amateur, ship, airline, etc.), and obliged the end users to choose compliant products. With a growing number of illegal products sold on the market, however, the government has started warning and disclosing the information of the manufactures or importers handling those incompliant products. Thus, the corporate stance and attitude for compliance are now being challenged and may influence customers' decision making.
Contact Us for Any Questions
お気軽にお問合せください

Call Us at お電話でのお問合せ
Contact us 24 hours via the form below.
問合せフォームは24時間受け付けております。お気軽にご連絡ください。
Updates 新着情報
2025年10月12日の輸入申告項目の追加に従い、記載内容を一部更新しました。
2023年10月1日の関税法基本通達改正に従い、記載内容を一部更新しました。
Our column page “Customs Specialist Eyes” is updated.
コラムを更新しました。
Our column page “Customs Specialist Eyes” is updated.
コラムを更新しました。
We’re now on Amazon SPN/Service Provider Network.
当ページ運営会社が、Amazon SPN(サービスプロバイダーネットワーク)に登録されました。
ホームページを公開しました